From tiny screws to automotive engine components, manufacturers coat even the inner surface of your phone’s casing with an ‘invisible armor’—a layer of nickel plating.
Though inconspicuous, it remains an indispensable surface treatment technology in modern industry.
Today, we explore the mysteries of nickel coating: its applications, unique advantages, and challenges.
Nickel Plating: Cladding Metals/Non-Metals in Nickel
Simply put, nickel coating involves uniformly applying a layer of nickel or nickel alloys onto an object’s surface through electrolysis (electroplating) or chemical reactions (chemical plating).
Which materials can be “clad” in nickel?
Common metals: Steel (the dominant choice), copper and its alloys (brass, bronze), aluminum alloys, zinc alloys, stainless steel.
Non-metals can also be coated: Plastics (e.g., ABS, PC/ABS commonly used in phone cases), ceramics (requires a chemical plating base coat before electroplating).
Different “Plating Solution Formulations”:
Watt nickel (economical choice): Primarily nickel sulfate, most widely used, low cost, moderate hardness, preferred for decorative and basic protective coatings.
Aminosulfonic acid nickel (low-stress high-performance): Low internal stress, excellent toughness, suitable for precision molds and electroformed parts.
Chloride Coating (Repair Specialist): Offers rapid deposition and excellent conductivity, commonly used for quick repair of worn parts.
Citrate Coating (Eco-Friendly Mild Option): Better suited for sensitive materials like aluminum and zinc, supporting environmentally friendly practices.
“Nickel Coatings” Serve Diverse Functions:
Bright Nickel: Incorporates special brighteners to achieve a mirror-like finish directly, delivering exceptional luster.
Semi-Bright Nickel: A low-sulfur “powerhouse” offering superior corrosion resistance compared to bright nickel.
Nickel Sealer (The Team Player): Specifically designed for subsequent chrome plating, forming micro-pores that significantly enhance overall corrosion resistance.
Compound Coating (Hardcore Edition): Incorporates SiC, diamond, or PTFE particles for enhanced wear resistance or self-lubrication.
Nickel Plating’s “Ace in the Hole”: Why It’s So Favored?
Rust and Corrosion Resistance “Golden Shield”:
Nickel resists air, water, and alkalis. Multi-layer plating exceeds 500 hours salt-spray resistance, ideal for harsh environments.
The Aesthetic Star “Polished Mirror”: Bright nickel layers boast mirror-like brilliance.
Faucets, automotive trim, door handles, and more rely on it to elevate product quality.
Tough and Durable “Hard Bone”:
Typically hardening to 200–500 HV (Vickers hardness), far exceeding copper-zinc coatings.
Heat treatment can push it to 1000 HV, significantly boosting part longevity.
Reliable “Base for Electronic Soldering”:
Pure nickel provides a stable, dependable surface for soldering, widely used in electronic connectors and terminals.
Electromagnetic Interference Shielding: Nickel’s conductivity makes it an excellent electromagnetic shielding material.
The thin electroless nickel layer inside your phone’s plastic case quietly shields against interference signals.
Versatile Coverage: No job is too challenging.
Regardless of shape complexity (deep holes, grooves), uniform coverage is achievable through pre-plating or electroless plating.
The “Headaches” of Nickel Plating: Challenges and Disadvantages
Cost is a “Minor Pain Point”:
Nickel prices fluctuate significantly (around $15,000–20,000/ton), making overall process costs higher than zinc plating and similar methods.
Environmental Pressure as a “Tightening Restriction”: Plating solutions contain toxic nickel ions and additives like formaldehyde.
Wastewater treatment must strictly meet standards, and environmental regulations are increasingly stringent.
Porosity as a “Small Loophole”: Single-layer coatings may exhibit microscopic pores, allowing corrosion media to penetrate.
This requires multi-layer systems or denser coatings for resolution.
Uniformity Challenge: Deep holes and recesses thin the plating, so engineers use auxiliary anodes or pulse plating.
Internal Stress “Troublemaker”: Some bright nickel layers contain sulfur, generating high internal stress that may cause brittle fracture or flaking.
Low-stress baths like amino sulfonate formulations mitigate this issue.
Nickel Plating: Showcasing Its Versatility Across Endless Applications
Reliable Protection for Vehicles: Brake lines, radiators: Prevent corrosion from coolant.
Door handles, emblems: Bright nickel paired with chrome enhances aesthetic appeal.
Piston rings: Composite coating (e.g., with SiC additives) greatly improves wear resistance.
Invisible Shield for Electronics: SIM card slots, USB ports: Nickel base plating prevents oxidation, followed by gold plating for conductivity.
Battery electrodes: Nickel foil serves as a critical current collector in lithium batteries.
Routers, computer cases (plastic): Chemically deposited nickel layers provide essential electromagnetic shielding.
Durable foundation for mechanical engineering: Hydraulic rods, molds: Nickel plating ≥100μm thickness delivers exceptional wear resistance.
Worn bearings and shafts: Electroplated nickel precisely restores dimensions, saving material and costs.
Refined care for home living: Faucets and showerheads: Multi-layer nickel-chrome structures resist moisture corrosion, maintaining lasting luster.
Cutlery (knives, forks, etc.): Food-grade nickel coating compliant with ISO 4531 and other standards ensures safety and aesthetics.
High-temperature armor for aviation:
Aircraft engine components: Nickel-alloy plating (e.g., Ni-Co) resists high-temperature oxidation.
Innovative partner for cutting-edge fields:
3D-printed parts: Nickel coating fills surface pores, improving strength and airtightness.
Fuel cell bipolar plates: Nickel coating prevents hydrogen embrittlement, ensuring safe and efficient operation.
Core Insights: Detailed Explanation of Nickel Plating Process (Key Steps!)
Applying a flawless nickel coating demands more than immersion—it requires precise electroplating or chemical plating, with key steps as follows:
Pre-treatment (Critical to Success!):
Degreasing: Thoroughly remove surface oils using alkaline solutions or solvents (otherwise, the coating may peel or bubble).
Pickling and Activation: Use hydrochloric acid, sulfuric acid, etc., to remove rust and oxide scale, exposing fresh metal surfaces to enhance adhesion.
Rinsing: Thoroughly rinse after each chemical step to prevent solution cross-contamination.
Special Substrate Treatments:
Steel: Often requires copper or nickel pre-plating as a base layer (to prevent displacement reactions).
Aluminum/Zinc Alloys: Requires zinc immersion or special activation treatment.
Plastics: Requires roughening, sensitization, and activation (enabling surface adsorption of catalytic metals like palladium).
Nickel Plating (Core Process): Electrolytic Nickel Plating (Mainstream, Requires Electrification):
Immersion: Submerge prepared workpieces (cathodes) and nickel anodes into the nickel plating bath.
Electrodeposition: Apply direct current.
Nickel ions reduce to metallic nickel atoms at the cathode (workpiece) surface upon electron gain, depositing layer by layer.
Strict control of current density, temperature, pH, and time is essential.
Plating bath type determines properties: Select Watt’s solution, amino sulfonate solution, etc., as needed (see Part I).
Additive Functions: Brighteners, leveling agents, wetting agents, etc., control coating gloss, flatness, and porosity.
Electroless Nickel Coating (Autocatalytic, No Electric Current):
Immersion: Immerse the activated workpiece into a solution containing nickel salts (e.g., nickel sulfate) and a reducing agent (e.g., sodium hypophosphite).
Autocatalytic Deposition: On a palladium catalyst, the reducing agent converts nickel ions to nickel and deposits phosphorus, forming a Ni-P alloy.
The reaction proceeds autonomously, yielding uniform coatings (without edge effects) suitable for complex geometries and substrates like plastics.
Post-Treatment (Enhancing Performance and Appearance):
Rinsing: Thoroughly remove residual plating solution.
Passivation: The solution forms a protective film on nickel, boosting corrosion resistance (optional blue-white or black color).
Sealing: For coatings requiring pore sealing (e.g., nickel sealing) or additional protection, apply oil immersion, wax coating, or organic sealants.
Heat Treatment: (For chemical nickel-phosphorus plating) Heating (typically 260°C–400°C) substantially increases hardness and wear resistance.
Chromium Plating/Other: For high decorative requirements, apply decorative chrome or hard chrome over bright nickel.
Drying: Thoroughly bake to prevent corrosion from water marks or residual moisture.
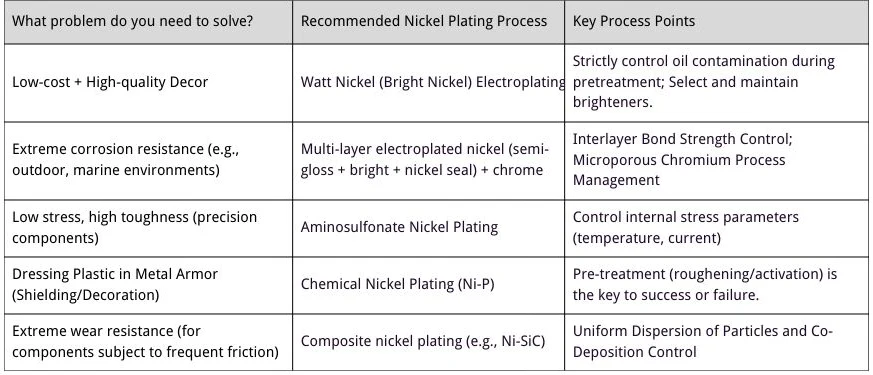
How to Choose the Right Nickel Plating Process? One Chart to Guide Your Decision!
Future Trends: Greener, Stronger, Smarter
Green Upgrade: Cyanide-free pre-copper plating and eco-friendly trivalent chromium passivation are gradually replacing high-pollution processes.
Performance Leap: Nanocrystalline nickel coatings exceed 600HV hardness, meeting precision instrument demands;
Alloy coatings like Ni-P (electroless nickel), Ni-W, and Ni-Mo demonstrate superior high-temperature resistance and corrosion resistance.
Smart Integration: Combining automation with online monitoring technology enhances process stability and efficiency.
Conclusion
Nickel plating, a traditional surface treatment, continues evolving through innovations in high-end manufacturing, green tech, and daily life.
It enhances a product’s look and durability while protecting its performance and longevity.
Next time you see gleaming hardware or electronics, remember the nickel coating beneath—the invisible armor of modern industry.
FAQ:
Nickel plating is the process of coating a metal or non-metal surface with a thin layer of nickel through electroplating or chemical deposition. This “invisible armor” enhances corrosion resistance, wear resistance, and conductivity, making it indispensable in electronics, automotive, and consumer goods.
Nickel plating works on a variety of substrates including metals like steel, copper, brass, aluminum alloys, zinc alloys, stainless steel, and non-metals such as plastics and ceramics (with special pre-treatment).
Common formulations include Watt’s nickel (economical), aminosulfonic acid nickel (low-stress, high-performance), chloride coatings (rapid repair), and citrate coatings (eco-friendly for sensitive materials). Each solution offers unique hardness, stress levels, and environmental compatibility.
Nickel plating provides exceptional corrosion resistance, mirror-like aesthetics, high hardness (200–500 HV, up to 1000 HV with heat treatment), reliable electronic soldering surfaces, and electromagnetic interference shielding.
Key challenges include fluctuating nickel costs, environmental regulations due to toxic plating solutions, microscopic porosity, non-uniform coverage in complex geometries, and internal stress in some bright nickel coatings that may cause flaking or brittleness.
Nickel plating protects electronic components like SIM card slots, USB ports, battery electrodes, and plastic cases. It prevents oxidation and provides a stable, conductive base for further gold or copper plating, while also serving as an EMI shield.
Industries ranging from automotive (brake lines, radiators, piston rings), aerospace (engine components, high-temperature oxidation protection), mechanical engineering (hydraulic rods, molds), to home appliances (faucets, cutlery) and cutting-edge tech (3D-printed parts, fuel cell bipolar plates) rely on nickel plating.
Electrolytic nickel plating requires an electric current for deposition, while electroless (autocatalytic) plating uses a chemical reaction to deposit Ni-P alloy uniformly, even on complex geometries or non-metal substrates like plastics.
Performance is improved through pre-treatment (degreasing, pickling, activation), post-treatment (passivation, sealing, heat treatment), multi-layer coatings, and additives such as brighteners, leveling agents, or embedded particles (SiC, diamond, PTFE) for wear resistance or self-lubrication.
Nickel plating is evolving toward greener, smarter, and higher-performance solutions: eco-friendly cyanide-free pre-treatments, nanocrystalline or alloy coatings (Ni-P, Ni-W, Ni-Mo) for superior hardness and corrosion resistance, and automated, monitored processes for consistent quality.