Medical applications of rapid prototyping technology
Someone developed rapid prototyping (RP or RPM) technology abroad in the late 1980s.
It integrates CAD/CAM, CNC, laser processing, automatic control, and new materials science; its basic principle is:
The computer slices a 3D model and guides a laser or nozzle to layer and fuse materials into a prototype.
Rapid prototyping technology has the following characteristics:
- Does not require any tooling, molds and fixtures;
- STL format file to directly accept the three-dimensional CAD solid model data;
- The use of “layered manufacturing, layer by layer” principle;
- Rapid and direct manufacturing of any complex shape of the solid sample or mold;
- The entire manufacturing process is automatic.
Rapid prototyping cuts development to under a week, vital for medical prostheses from CT data to implantation.
Engineer integration into medicine has driven widespread RP use in the past decade.
After nearly a decade of R&D, current medical applications of Rapid Prototyping include:
Prosthesis fabrication
Researchers used STL files from CT data to fabricate a cranial defect model via SLA with 0.354mm slices.
The 3 mm CT layer thickness limits overall accuracy. fabrication took 18 hours.
Technicians filled the model with wax and molded it in plaster to make a well-fitted titanium prosthesis, improving clinical outcomes.
To cut RP costs, engineers reconstruct only the defect area for titanium plates, saving time and money with excellent results.
RP enables implant design and fabrication from patient-specific CT or MRI data instead of standard anatomy.
This reduces implant design errors and ensures better anatomical fit, improving surgical outcomes.
Custom implants help surgeons reduce operating time significantly.
This shortens anesthesia time, improves aesthetics, and cuts costs and complications.
Surgical Programming
Someone applied the SLA model to surgical planning in the United States in 1992.
The patient’s variable cleft palate required a plastic model before orthopedic surgery.
The SLA model from CT images displays both internal and external, including concave, structures.
This SLA model allows the surgeon to plan the surgery and to anticipate the outcome of the surgery at first sight.
In maxillofacial surgery, SLA models help surgeons plan procedures before surgery.
For two mandibular surgeries, SLA models enabled surgeons to pre-design plans, paths, and prepare instruments.
The authors don’t detail model manufacturing, suggesting possible remote production by the company.
They report a 1mm CT data error and suggest tooth occlusion accuracy must be within millimeters.
Bioengineering
Researchers used rapid prototyping in bioengineering to create an adult femur model for testing a new artificial hip joint.
After CT imaging, authors processed and converted data to STL to offset sintering shrinkage.
Someone used a two-part cranial model for accuracy checking simultaneously.
The Sinterstation (DTM Corporation) molding machine sintered the model with nylon powder.
The rapid prototyping layer was 0.1 mm thick, with fabrication taking 12 to 48 hours based on powder injection.
Other applications
RP is used for other non-osteosynthesis prostheses.
They make a breast model by inverting normal breast CT images for surgeries like breast removal.
Prosthesis fabrication uses CT data converted to STL and CAD with finite element analysis to optimize fit and load.
The team fabricated a well-fitting prosthesis from polycarbonate using the SLS method.
This method ensures perfect prosthesis fit and proper weight-bearing stress distribution.
RP technology achieves a good fit while simplifying and speeding up the manual procedure.
Data from 45 craniomaxillofacial surgery patients show RP models greatly improved diagnosis and surgical planning.
Biomimetic modeling raised diagnostic accuracy to 95.2%, reduced error to 8%, and greatly shortened surgery time.
Rapid prototyping enables fast, remote, personalized prosthesis production for tumor and deformity correction.
CT image inversion enables rapid prototyping design and manufacturing.
CT image inverse request technology is mainly divided into the following steps;
Data Acquisition
CT images are acquired, stored as grayscale matrices, and sent to a microcomputer for processing.
Edge enhancement (also known as sharpening)
Image analysis shows sharpening enhances high-frequency edge details over low-frequency areas.
Edge tracking
CT images are binarized from grayscale using histogram-based thresholding.
Set the background color to black using the 3×3 template, expressing its grayscale as 1.
the tissue is white and is expressed as 0.
Contour Line Approximation and 3D Reconstruction
To reduce redundant contour data, researchers extract feature points using common methods such as:
Isometric sampling causes the loss of many feature data points.
Curvature sampling extracts feature points via second derivatives, requiring contour-specific curvature range and max spacing T.
The geometric chord method approximates by setting the minimum point-to-chord distance for accuracy.
The last method is highlighted below.
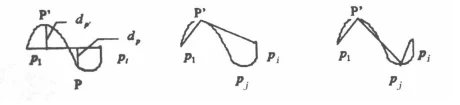
Any curve arc can be approximated by chords, with accuracy defined by the maximum point-to-chord distance.
Figure 1 shows the arc p1piP and defines dp as the distance from any point P on the arc to the chord p1pi.
dp′ = max| dp| p ∈ p1pi
Procedure: Set a threshold d; approximate arc p1pi with string p1pi; if the max distance dp′ ≤ d, accuracy is met.
If not, split arc p1pi into p1p′ and p′pi, approximate each, and repeat until dp′ ≤ d (see Figure 1).
Then match inter-layer feature points by finding corresponding points between contour lines.
Commonly used methods are:
- Based on a certain shape metric criterion (e.g. length, surface area, volume, etc.);
- Utilizing syntactic pattern matching methods;
- Matching methods based on shape feature points and matching algorithms based on CT image properties.
Transformation into a form acceptable to the RP system
- Generate STL files for direct use by the data processing software of the RP system.
- CLI files directly drive RP to stack layered contours into 3D objects, a key research area.
- Reconstruct the 3D CAD model into STL file with the help of CAD system.
Design Example
CT scans capture grayscale layer maps of the lesion’s skeleton at set intervals.
CorelDraw vectorized grayscale contours to accurately reproduce each bone layer’s outer and inner shapes.
CAD/CAM software reconstructs data points into curves, surfaces, and solids for 3D reconstruction.
Pro/E’s SCAN MODEL processes input data to obtain inner and outer contour lines for each layer (Fig. 2).
Curves form surfaces that create a closed solid with fine tetrahedral mesh, output as STL files for rapid prototyping.

The rapid prototyping machine slices STL solids into contour layers;
the workstation optimizes laser paths and sends them to the machine for segmented production.
The surgeon refers to the solid model of the lesion when developing the surgical plan.
If necessary, the CT scanning area can also be expanded to include related tissues or organs.
CT imaging combined with rapid prototyping provides solid information for surgical procedures.
Rapid prototyping offers clear advantages by accurately depicting key structures near lesions or for postoperative evaluation.
Precise 3D reconstruction aids optimal surgical view and window selection, improving planning
FAQ:
Rapid Prototyping is a manufacturing process that creates physical models directly from 3D CAD data by layering materials without the need for molds or tooling. A laser or nozzle fuses material layer by layer based on sliced CAD models, typically in STL format.
RP integrates several advanced technologies including CAD/CAM, CNC, laser processing, automatic control systems, and new materials science.
Because it enables quick, personalized production of prostheses and surgical models from CT or MRI data, reducing errors, improving anatomical fit, shortening surgical times, and improving patient outcomes.
CT data is converted to STL files, then processed through methods like SLA or SLS to produce a solid model. This model is used to fabricate prostheses that perfectly fit the patient’s anatomy, often reducing time and cost.
RP produces detailed 3D models from medical imaging data, allowing surgeons to pre-visualize the procedure, simulate surgical paths, and prepare tools in advance, which helps reduce risks during surgery.
RP can produce cranial, femoral, mandibular, breast, and other anatomical models, supporting procedures like cleft palate surgery, hip replacements, and tumor removals..
Key steps include CT data acquisition, image enhancement and binarization, contour extraction and approximation, inter-layer point matching, 3D reconstruction, and conversion to STL or CLI files for RP manufacturing.
STL (stereolithography) is the most commonly used file format in RP, as it represents 3D geometry as a mesh of triangles and is directly compatible with most RP systems.
Accuracy depends on CT scan resolution and layer thickness. For example, models made from 3 mm CT slices and 0.354 mm SLA layers can achieve high anatomical fidelity. Some errors may occur due to data granularity, but precision can be improved with careful processing.
RP enables precise modeling for testing artificial joints, optimizing load-bearing design, reducing human error in manual fabrication, and supporting faster development and evaluation of medical devices.